My research explores food webs in aquatic ecosystems in order to understand how the biodiversity is structured in these systems, how energy flows through these systems, and how intrinsic (e.g., plant and insect genetics, genetic diversity) and extrinsic (e.g., human disturbance, climate change) factors influence the structure and function of these systems. One of the tools I use to study aquatic food webs is stable isotope analysis. For example, by isotopically labeling leaf litter, I am able to create tracers for carbon and nitrogen that reveal how energy moves from leaf litter to higher trophic levels.
Another approach I use to study food webs is to use high-resolution biodiversity data from DNA metabarcoding to create taxonomic lists of all of the organisms found in a system, and then to link these taxa using existing trait information mined from the literature. This allows me to create “heuristic food webs” which can be used to visualize the biodiversity in an ecosystem, understand how this biodiversity interacts, and make predictions about the health and resilience of these systems.
Stable Isotope Ecology

Marks (2019)
Isotopically Labeled Leaf litter
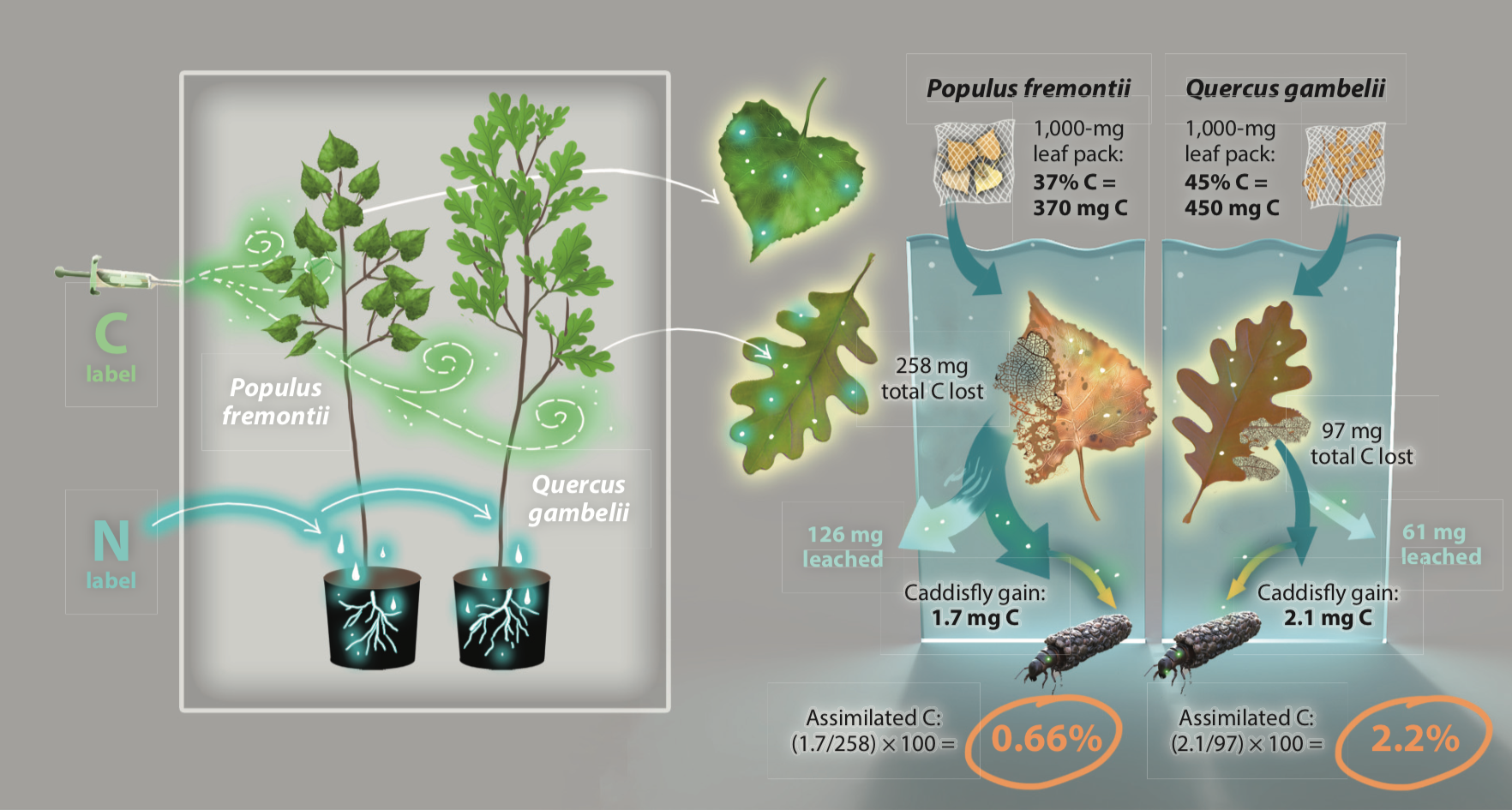
I label leaf litter with carbon and nitrogen to trace how it flows through aquatic food webs.
Slowly Decomposing Litter Supports the Macroscopic food web
Despite the fact that rapidly decomposing litter is often viewed as a “high quality” resource, it yields less carbon and nitrogen to stream shredders compared to slowly decomposing litter.
Marks (2019)
Rapidly Decomposing litter fuels the microbial food web
One of the reasons for these surprising patterns is the loss of carbon and nitrogen from rapidly decomposing leaf litter to leaching and microbial respiration. making it less available to aquatic insects and their predators. Slowly decomposing leaf litter, on the other hand, has more complex forms of carbon that bind nitrogen and allow it to persist longer in the stream after autumn senescence, making it more available to the macroscopic food web.
Implications for riparian food webs
These findings suggest that forests with fast decomposing leaf litter will not support higher trophic levels compared to forests that contain species and genotypes of slowly decomposing leaf litter, which can slow down decomposition in mixed litter systems. Collectively, this means that forests with diverse riparian tree assemblages likely lose less carbon to the microbial food web and produce more insects that support fish and terrestrial predators feeding at higher trophic levels.
Environmental
Genomics

Kennedy et al. 2020
Biomonitoring 2.0: rapid assessment of biodiversity in high-resolution
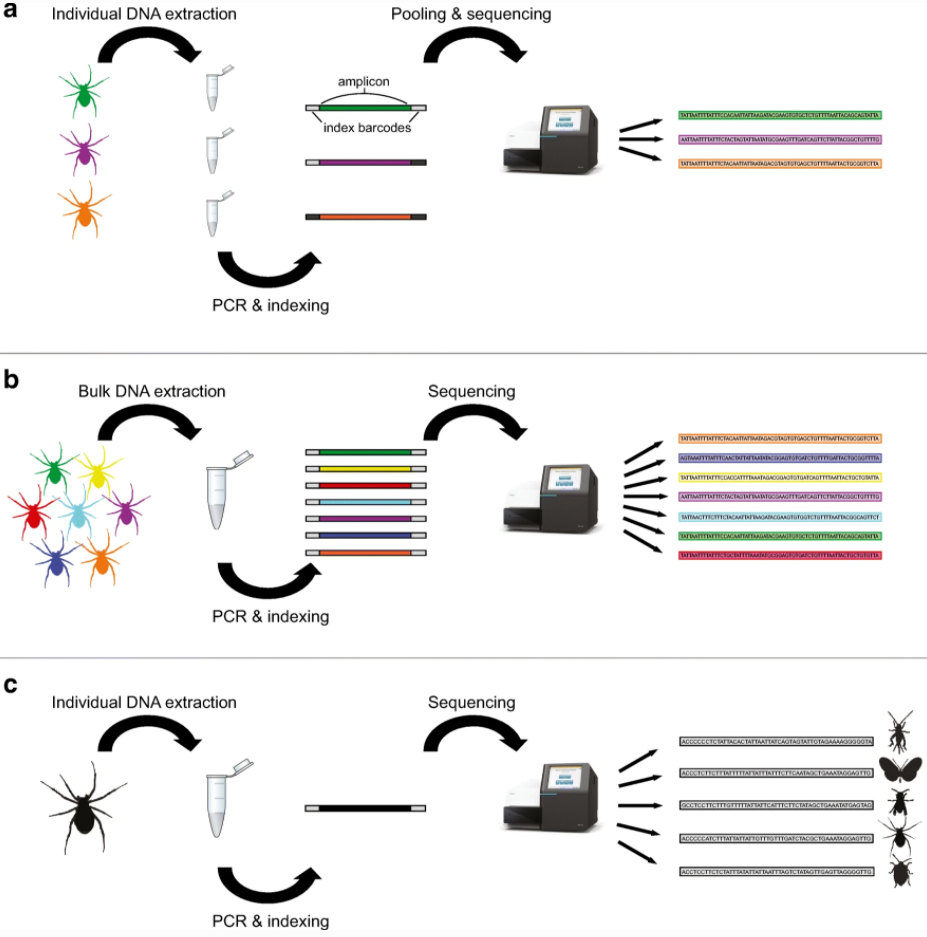
DNA is everywhere in the world around us: in the water, in the soil, even in the clouds. The field of environmental genomics explores ways of collecting this DNA and making sense of it. DNA can be found inside the bodies of living organisms, or outside of cells, where it originates from sloughed off, excreted, or decomposing plant, animal, or microbial cells and can persist in the environment for weeks to months. This loose DNA floating around in the environment is called “environmental DNA,” or “eDNA.”
Gibson et al. 2015
Traditionally, biodiversity is assessed by collecting organisms in the environment. Collecting the samples comes at incredible cost, in terms of time and money, especially in remote and hard to reach areas (left, cegacanada.com). Once samples are collected, there is an additional cost in terms of the substantial amount of time and taxonomic expertise required to count and identify the organisms in these samples. In a 0.5 L bottle of water from a wetland, for example, there can be hundreds or even thousands of organisms! Consequently, this type of sampling can limit the capacity to monitor environments in space and time, and compromises are often made, including reducing sampling coverage and assessing only a fraction of the organisms in a sample. Moreover, maintaining consistency in observing biodiversity across regions and systems is difficult, given the different sampling approaches and varying levels of taxonomic expertise among organizations and governing districts.
The environmental genomics approach, which has been called “Biomonitoring 2.0” or “Next-Generation Biomonitoring", uses a revolutionary technology called DNA metabarcoding in order to assess the entire complement of biodiveristy in a single sample. This approach takes fragments of eDNA floating around in the environment and matches them to DNA barcodes available in genomic databases online. In a single water sample that looks clear to the naked eye, DNA metabarcoding can rapidly uncover hundreds of species and tens of thousands of unique fragments of DNA. While traditional biomonitoring often focuses on a single group of organisms or “indicator taxa,” DNA metabarcoding can assess all of the organisms with DNA in the sample, from microbes to whales. The applications for this technology are tremendous—from rare or invasive species detection to environmental forensics—and when this rich DNA information is coupled with other information, our lab uses it to reconstruct food webs for the entire ecosystem sampled!
Ecological Network Analysis

Social network graph (image credit: Annalie Kruseman).
When information exists about how entities in a community interact it is possible to visualize these interactions in a network graph. A classic example is a social network (left), where nodes depict people and lines depict interactions these people share on a social media site. Lines and nodes can be scaled based on the frequency or intensity of the interactions, illuminating key players in the network and dominant pathways of information transfer.
While information on human social networks is—for better and worse—widely collected and often publicly available, information on interacting species is often much harder to assess because there are thousands of species. First, all of these species must be detected in the system. Next, considerable effort (via direct observation or gut contents analysis) must be invested to determine how these species interact. Because of these challenges, relatively few well-studied food webs are published in the scientific literature. Instead, many scientists have attempted to mathematically model how species interact based their prevalence, allowing them to predict the probability that species will interact in an ecosystem.
Heuristic food web of the Dog River, Northwest Territories, Canada. Primary producers are green circles, and consumers are blue squares. Taxa are plotted according to their trophic level (y-axis).
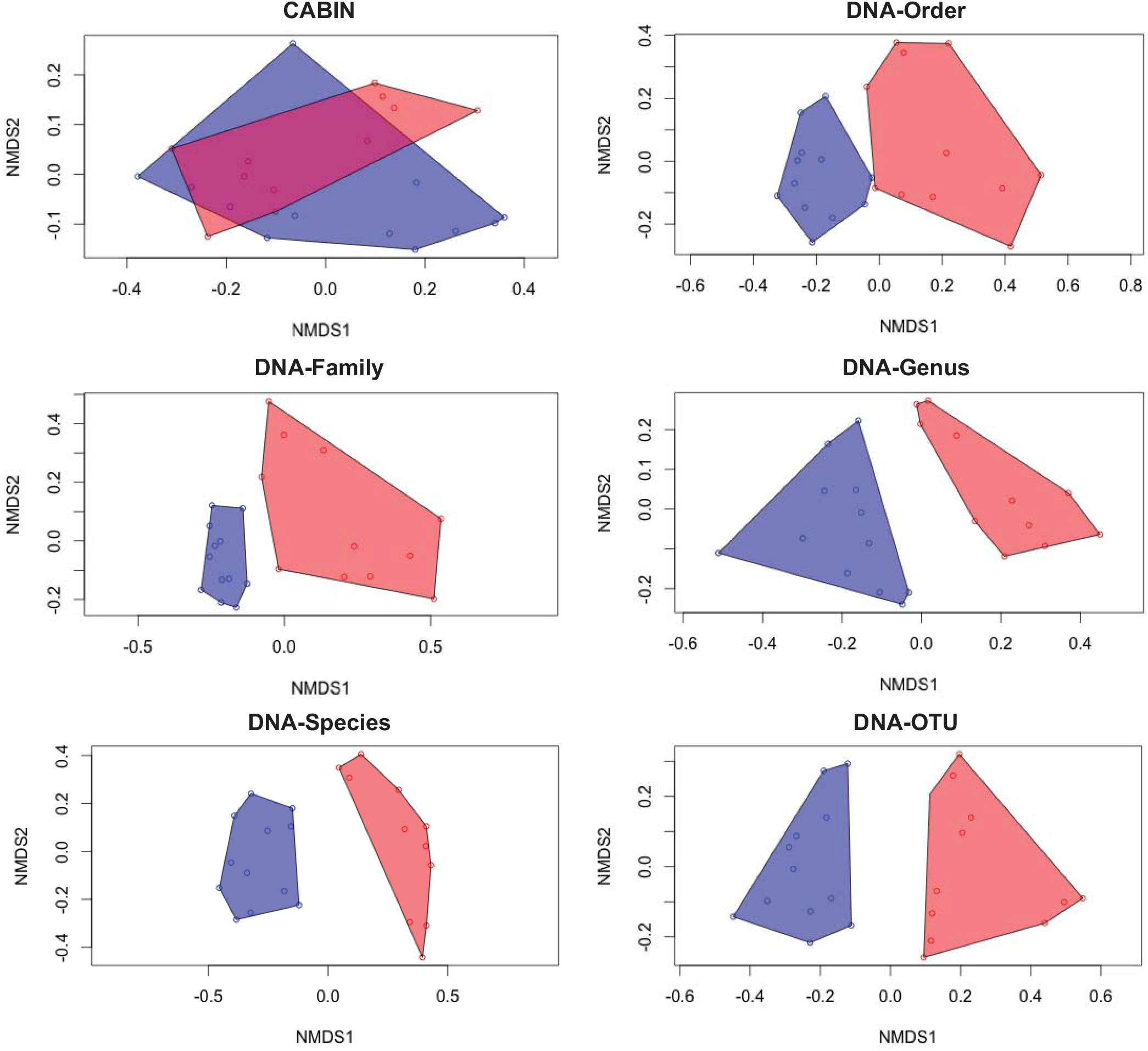
By matching taxa with documented co-occurrence information (found in the scientific literature or trophic linkage databases) it is possible to further structure the ecological network to include trophic position (right). These are hypothetical, or “heuristic” food webs. Because they are grounded in empirical evidence collected from the literature, they are more realistic than theoretical mathematical models of food webs. My work has demonstrated that these heuristic food webs have properties (e.g., linkage density, connectance, trophic position) that are sensitive to spatial and temporal patterns in aquatic wetlands. Additionally, recent work in our lab has demonstrated that the trophic position predicted for organisms in these food webs reflects their actual trophic position (measured by stable isotope analysis).
Because some of the properties of food webs (e.g., maximum trophic position and linkage density) can reflect the fragility of food webs (Cohen et al. 2003; Jonsson et al. 2005), it is possible that heuristic food webs could become a powerful tool for rapid bioassessment, allowing users to assess the relative health of aquatic ecosystems and providing early indicators of ecosystem impairment.
Common Gardens and Climate Change

Illustration by Victor Leshyk.
www.sega.nau.edu
Common Gardens as a Tool to study climate change
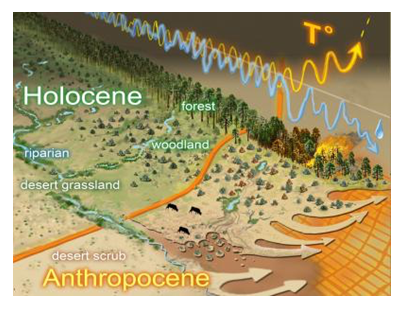
Climate change is expected to affect nearly all aspects of natural systems (above, left). Common gardens are a powerful tool for disentangling how plant genetics and the environment interact to affect ecosystems. By planting trees with known genetics in a common environment, this reduces the environmental influence on associated communities and ecosystem functions. When gardens with the same tree genotypes are replicated and planted in many different environments (e.g., different elevations, above, right), these gardens can assess how warming is likely to influence these trees, their associated communtiies, and the functions these communities perform, therefore serving as a critical tool for understanding how climate change will affect terrestrial and aquatic ecosystems.
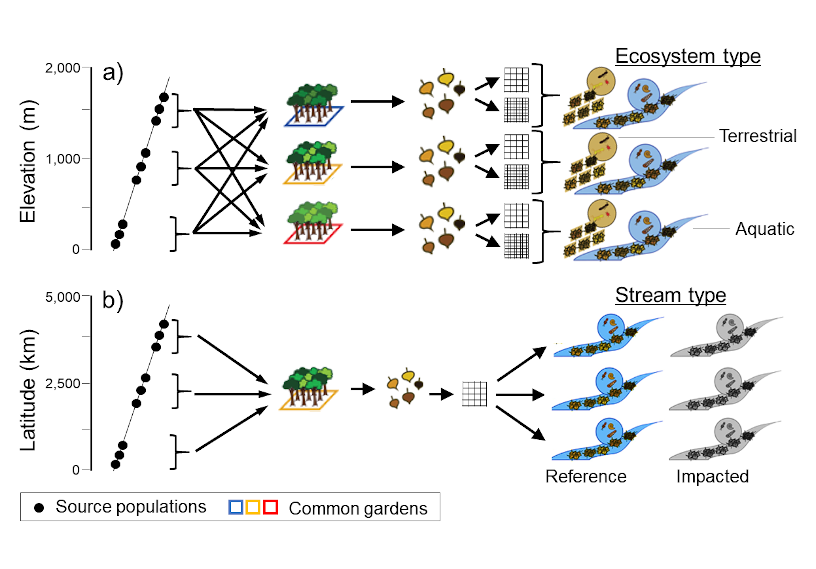
leaf litter from common gardens can reveal dynamic patterns
Collecting leaf litter from common gardens that include tree genotypes from different elevations (a) and lattitudes (b) makes it possible to explore how plant genes govern ecosystem processes in terrestrial and aquatic ecosystems, and illuminates questions about the influence of genetic diversity on associated tree communities and the functions they perform. Are aquatic insect communities locally adapted to leaf litter from trees of local genotypes? How does plant genetic diversity influence energy movement through food webs and across ecosystems (e.g., aquatic-terrestrial linkages)? How do climate change and tree genetics interact to influence aquatic communities and ecosystem functions, such as nutrient spiraling and litter decomposition?
PBS Documentary on the genes-to-ecosystem approach
Some of the research of The Cottonwood Ecology Group at Northern Arizona University has been featured in the PBS documentary, “A Thousand Invisible Cords,” including my research on how plant genetics influences the emerging aquatic insect community.